Did you know the metal structure can protect from corrosion by corroding another metal?
siddarthsparkle@gmail.com
November 15, 2024
📌As you aware from my previous posts, corrosion can happen only at anode, so automatically there is no any corrosion reaction at Cathode.
📌Metal structures have both Cathodic and Anodic areas. If all Anodic areas are converted to cathodic areas, the entire metal becomes a cathode.
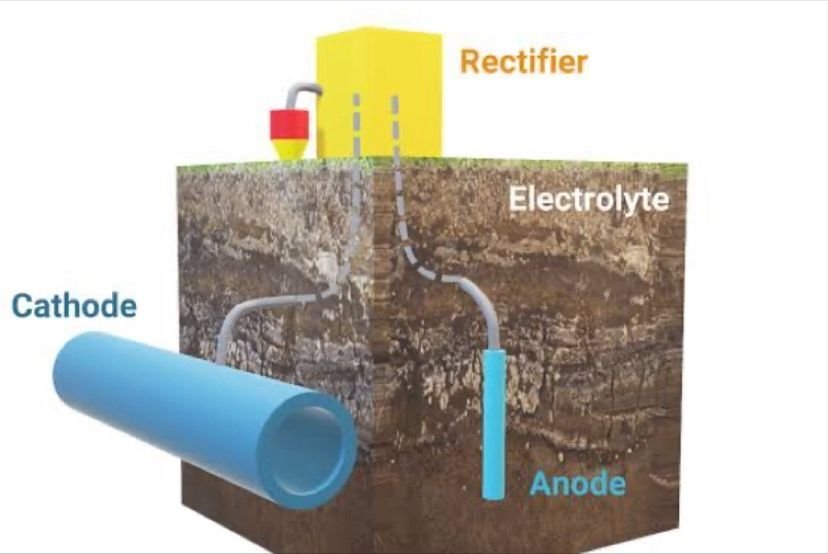
📌Turning the protected metal into a cathode by either impressed current or attached with galvanic anode can eliminate the corrosion.
📌The process called Cathodic protection, this is one of the best methods for preventing corrosion on metal surfaces
📌Use galvanic anode such as Magnesium, aluminium, or zinc act as anode against the protected metal and those anode get corrode to protect the metal(cathode)from corrosion.
📌Cathodic protection generally used in immersed or buried (moist underground) services, however, has protected thousands of miles of pipe and acres of steel surface subject to buried or immersed conditions.
📌This mechanism will not operate properly for above ground service. Protective Coatings is suitable one for above ground services.
🚶🚶♀️Follow me on more updates about corrosion control mechanisms.
