Do you know why Zinc is more reliable anode in Electroplating process?
siddarthsparkle@gmail.com
November 15, 2024
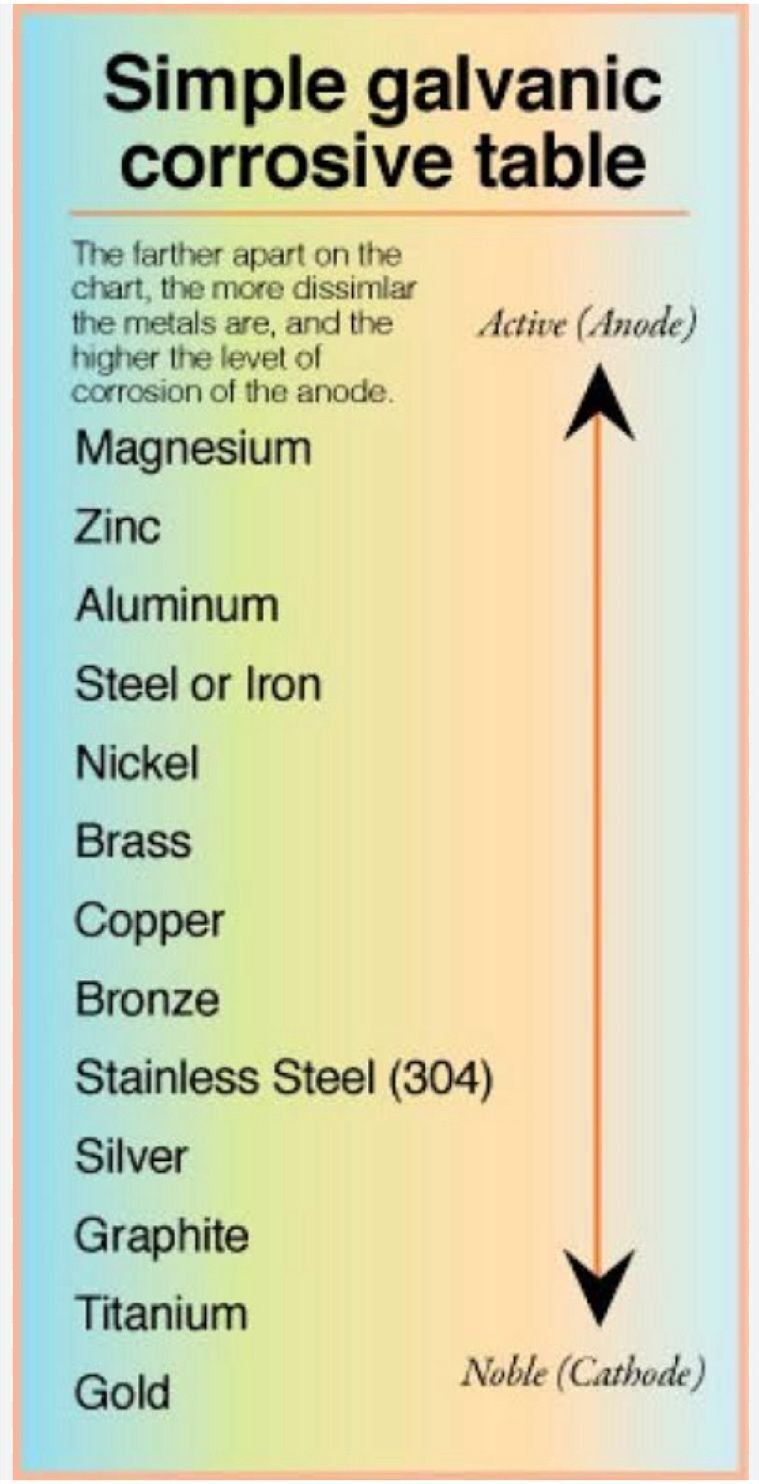
📌Zinc metal is more active metal in galvanic series and it’s oxides are preventing the corrosion reaction.
📌Zinc is more reactive than steel, corrosion rate of zinc is at least 10 times slower than steel.
📌Zinc corrodes first and protecting the steel substrate. Also it forms thin oxide layer to prevent further oxidation reactions.
📌Zinc is inexpensive metal comparatively any other metals in Galvanic series
📌It’s easy to passivate zinc plated parts to increase the protection.
📌Based on applications it’s used in various coating categories, details given below.
📍Sacrificial Coating
📍Decorative Coating
📍Functional Coating
📍Metal Coating
📍Alloy Coating
